-
日本語
-
English
PRODUCTS
Human Pancreatic Cancer Organoid Transplant Mouse
What is Human Pancreatic Cancer Organoid Transplant Mouse?
Human pancreatic cancer organoid transplanted mice are very similar to human pancreatic cancer tissues in clinical practice. This mouse model supports high-throughput and enables evaluation of drug efficacy of new drugs in a short period of time.
Background
Development of new drugs with stronger anti-tumor effects than the current ones, which is desired by the clinical field.
40 thousand people die annually from pancreatic cancer, and the 5-year survival rate for pancreatic cancer is extremely low at 9%. In order to improve this low 5-year survival rate, the development of new drugs with stronger anti-tumor effects than currently available drugs is needed in clinical practice.
In new drug discovery, it is necessary to evaluate drug efficacy using mouse models of cancer with a high degree of accuracy. However, in the case of pancreatic cancer, there are only a limited number of mouse models that can form tumors with a tissue architecture similar to human pancreatic cancer tissue, and there are no useful mouse models that can be used for high-throughput drug efficacy evaluation.
Overview
At Last, a Mouse Model with Tissue Architecture Extremely Similar to Human Pancreatic Cancer Tissue in Clinical Practice
This time, we have succeeded in establishing “human pancreatic cancer organoid transplantation mice” in which human pancreatic cancer organoids are transplanted subcutaneously into mice using the organoid culture technology to culture human pancreatic cancer cell lines in three dimensions. The pancreatic cancer tumors formed in this mouse model are extremely similar to those of human pancreatic cancer in clinical practice, making it an epoch-making mouse model that enables high-throughput drug efficacy evaluation of new drugs.
The “human pancreatic cancer organoid transplantation mouse,” which can provide reliable experimental results in a short period of time, meets the needs of researchers in academia and industry.
Characteristics
As a result of detailed investigation of conditions for creation of human pancreatic cancer organoids and establishment of a technique for creation of human pancreatic cancer organoids by overnight normal culture in an incubator, we have succeeded in homogenization of the pancreatic cancer cell growth speed (= increase in tumor volume) among individuals in nude mice implanted subcutaneously in the flanks.
The human pancreatic cancer tissue of this model shows an adenocarcinoma histology similar to that of clinical pancreatic cancer and is rich in carcinoma stroma, a characteristic feature of pancreatic cancer. In addition, it is possible to measure the concentration of CA19-9, a tumor marker, over time using mouse serum, thereby enhancing the evaluation of drug efficacy.
5 reasons for superior drug efficacy evaluation
01
High-throughput capability by using human pancreatic cancer cell lines

02
Mice can be supplied at 6 weeks from the initiated date cell growth

03
Able to conduct detailed examinations of toxicity to vital organs such as the lungs, liver, and kidneys using histopathological and blood tests.
04
Accurate evaluation of drug efficacy due to extremely small inter-individual variation in tumor volume

05
Measurable in this model serum because human pancreatic cancer cell lines express high levels of CA19-9

Characteristics deciphered by data
Human pancreatic cancer organoid culture technology
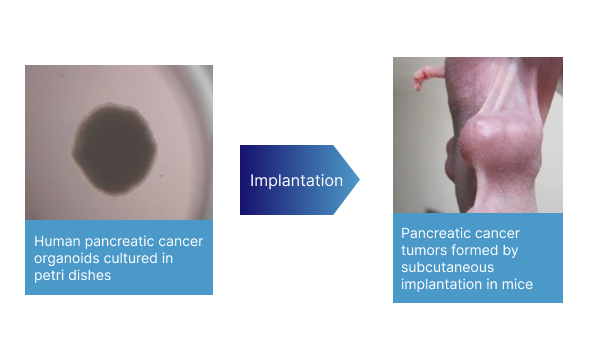
Organoid culture technology using human pancreatic cancer cell lines. When “human pancreatic cancer organoids” cultured using this breakthrough technology are transplanted subcutaneously into mice, “human pancreatic cancer organoid transplanted mice” can be supplied at 6 weeks from the day cell culture is started. The rapidity from cell culture to mouse supply is another feature of this mouse model.
Comparison with previous model Xenograft
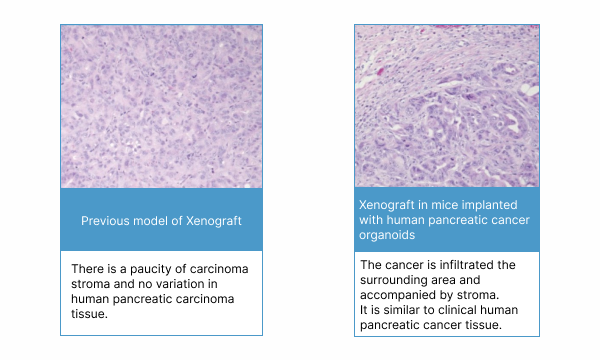
The pancreatic cancer tissue of this mouse model shows an adenocarcinoma histology similar to clinical human pancreatic cancer tissue and is rich in carcinoma stroma, a characteristic of pancreatic cancer. In addition, the concentration of CA19-9, a tumor marker, can be measured over time using mouse serum, which will enhance the evaluation of drug efficacy.
Experimental results of drug efficacy evaluation of GnP and GS therapies
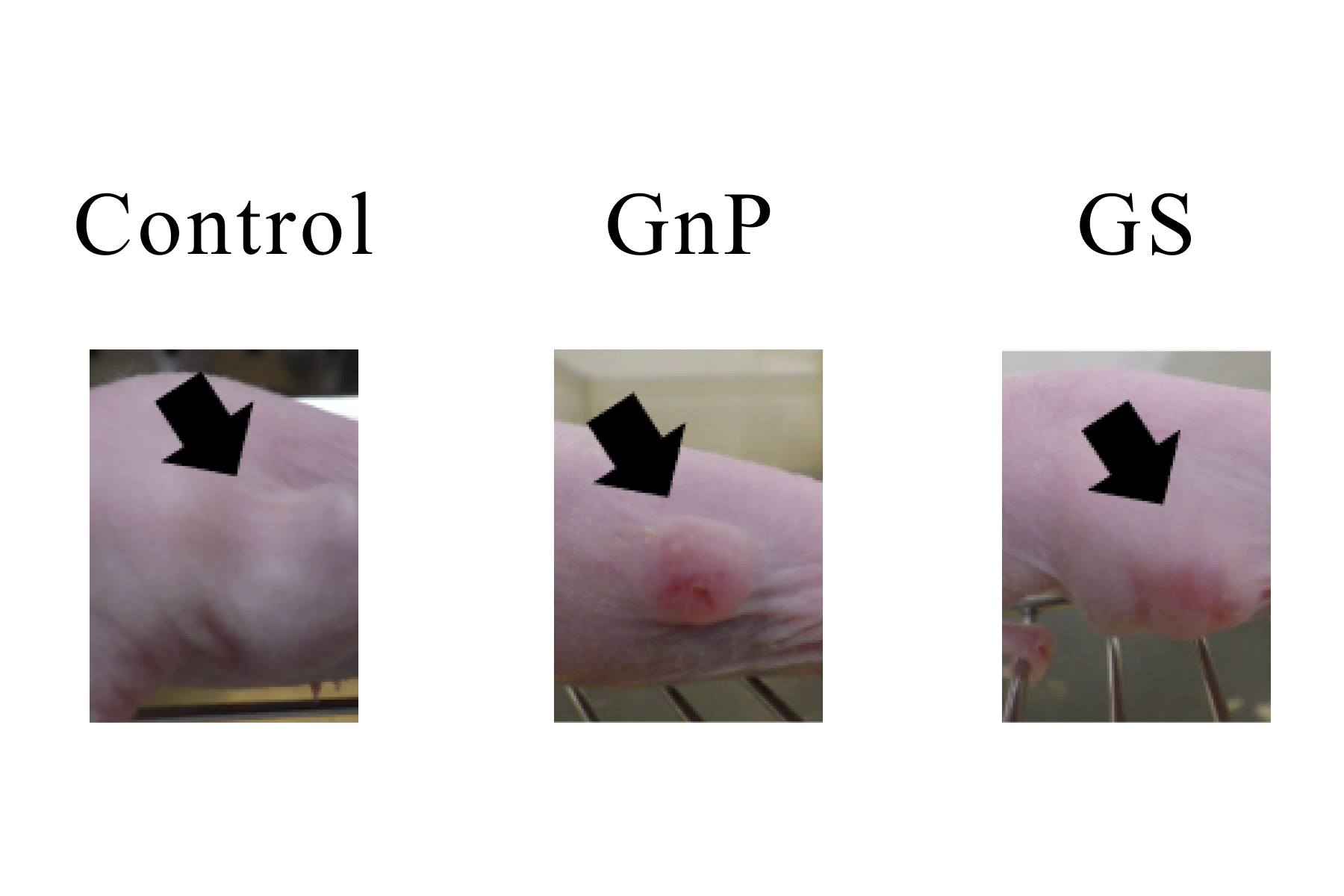
Mice transplanted with human pancreatic cancer organoids were divided into three groups (n=8 per group), and medication was started from the second week after the transplantation of human pancreatic cancer organoids under the flank skin of the mice, when the tumors had grown to some extent. The efficacy of gemcitabine was compared in a control group that received no medication, a group that received the combination of gemcitabine and nab-paclitaxel (GnP), which is recommended as the standard first-line chemotherapy for pancreatic cancer with distant metastases, and a group that received gemcitabine plus S-1 (GS), which has not demonstrated significant survival improvement compared with gemcitabine alone in previous trials. (GS), which did not show significant improvement in survival compared to gemcitabine alone in previous trials.
In the GnP group, gemcitabine (50 mg/kg) + paclitaxel (5 mg/kg) was administered intraperitoneally once/week for 3 consecutive weeks, with a 1-week break for 8 weeks. In the GS group, gemcitabine (50 mg/kg) was administered intraperitoneally once/week for 3 consecutive weeks with a 1-week rest period, and S-1 (10 mg/kg) was administered orally for 4 weeks with a 2-week rest period, repeated for 8 weeks. The GS group significantly suppressed the increase of pancreatic cancer tumors compared to the control group.
The results showed the superiority of GnP therapy, which is recommended as the standard primary chemotherapy for pancreatic cancer with distant metastasis in the guidelines for pancreatic cancer treatment (manuscript in preparation for submission).
Relative papers of “Human Pancreatic Cancer Organoid Transplantation in Mice”
1. Tanaka C, Furihata K, Naganuma S, Ogasawara M, Yoshioka R, Taniguchi H, Furihata M, Taniuchi K. Establishment of a mouse model of pancreatic cancer using human pancreatic cancer cell line S2-013-derived organoid. Hum Cell 35:735-744, 2022. doi: 10.1007/s13577-022-00684-7.
Establishment of a mouse model of pancreatic cancer using human pancreatic cancer cell line S2-013-derived organoid - PubMed (nih.gov)2. Preparing...